Electrolytic refining refers to the technology of extracting pure metals by utilizing the difference in the difficulty of anodic dissolution or cathodic precipitation of different elements. Electrolytic refining is commonly used in the refining of non-ferrous metals. Such as the refining of crude copper, crude silver, crude nickel, etc. During electrolysis, the crude metal obtained by high-temperature reduction is used to cast the anode, and the salt solution containing the metal to be prepared is used as the electrolyte, and a certain potential is controlled so that the impurities whose dissolution potential is higher than that of the refined metal remain in the anode or are deposited in the anode slime (which often contains precious metals), separated and recovered by other methods.
Impurities with a negative dissolution potential than the refining metal dissolve into the solution and do not precipitate on the cathode, so that refined high-purity metals can be obtained on the cathode. The metals refined by electrolysis include copper, gold, silver, platinum, nickel, iron, lead, antimony, tin, bismuth, etc. Through the electrolysis of the electrolyte solution, the crude metal is used as the anode, the pure metal is used as the cathode, and the solution containing the metal ions is used as the electrolyte, and the metal is dissolved from the anode and precipitated at the cathode. The impurities in the crude metal, the inactive impurities are not dissolved, and become anode slime and settle at the bottom of the electrolytic cell. Although the active impurities are dissolved in the anode, they cannot be precipitated in the cathode. Therefore, the metal with high purity can be obtained through the electrolysis cathode. It is called electrorefining of metals.
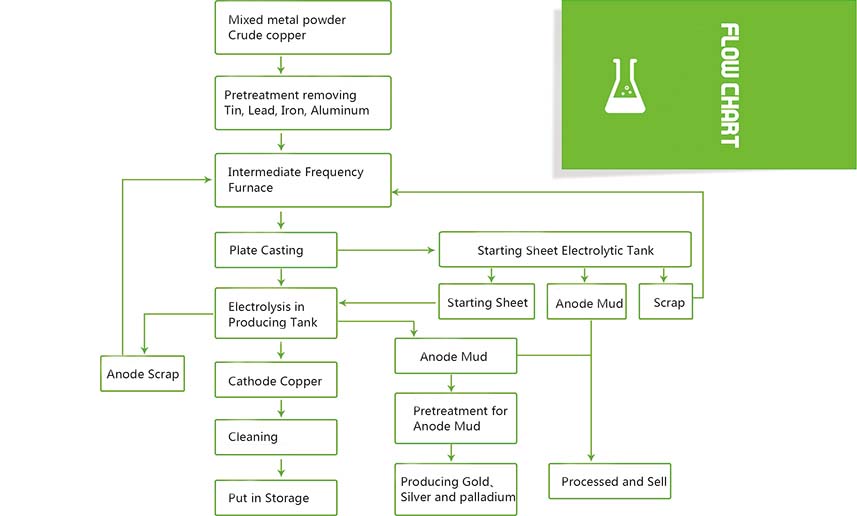
The product of copper pyrorefining is blister copper containing 99.0% to 99.6% copper. Due to its low purity, it cannot meet the requirements of industrialization. Therefore, it must be electrolytically refined to remove impurities that are difficult to remove in pyrotechnic refining. The pure copper sheet is used as the cathode, the cathode and anode are placed in the electrolytic cell, and the aqueous solution of sulfuric acid and copper sulfate is used as the electrolyte. Under the action of direct current, the copper on the anode dissolves and enters the solution, while the copper in the solution is on the cathode. Precipitate. In this process, the metal with negative potential than copper on the anode enters the solution, but cannot be precipitated on the cathode, and remains in the electrolyte to be removed during the purification process of the electrolyte; noble metals and some metals have a higher potential than copper dissolution potential due to their positive potential It is insoluble and precipitates at the bottom of the tank to become anode slime. In this way, the metal copper precipitated on the cathode is of high purity, which is called cathode copper or electric copper.
The main equipment of copper electrolytic refining:
(1) Electrolyzer
The electrolytic cell is the main equipment of the copper electrolysis workshop. It is a rectangular tank with anode plates and cathodes inside. There are liquid discharge outlet and mud discharge outlet in the tank.
The electrolytic cell is generally constructed of reinforced concrete and lined with anti-corrosion resin, so that the cell body can play the role of supporting the cathode and anode, and can also play the role of anti-acid. The electrolytic cells are arranged on the support beams in turn, and the beams are covered with insulating materials to prevent the cell body from conducting electricity with the ground.
(2) Cathode making unit
The function of this unit is to make a cathode by embossing and flattening the copper skin peeled from the titanium mother board, and then flattening the rivet ears (piercing rod), and then the cathode row spacing is prepared for the crane to lift it away.
Due to its unique function, the cathode manufacturing unit needs to be manufactured by a professional manufacturer. In the process of design and manufacture of the unit, the thickness of the copper skin and the processing capacity of the rivet ears should be considered.
(3) Anode processing unit
The function of the unit is to process the anode plate from the pyrolytic refining to meet the standards required by the electrolysis process. Each process of the unit is: flattening the anode plate surface, setting the lugs, pressing the lugs, and the row spacing of the anode plates.
In the design and manufacture of anode processing unit, the quality of each hydraulic seal and the processing capacity of anode plate should be fully considered.
(4) Electric copper washing unit
The function of the unit is to wash, dry, extract the conductive rod, stack, pack and weigh the copper after it has been discharged from the tank. Many domestic copper electrolysis manufacturers do not use this unit, and rely on manual work to complete the various functions of the unit.
(5) residual pole unit
The function of the unit is to wash, stack, pack, weigh, etc. the residual poles after they are out of the tank, and then send the packaged residual poles to pyrorefining and redissolving. Due to the simple function of this unit, many domestic copper electrolysis manufacturers do not use this unit, and rely on manual work to complete the treatment of residual poles.
Thank you for your interest in suny group. If you want to learn more about our E-waste recycling plant, copper wire recycling machine and other machines, Contact us now to find out what we can do for you next project!E-mail:sunymachine@gmail.com | Whatsapp:+8613674945231